how do ionic bonds form
Forming ionic bonds Positive and negative ions form when a metal reacts with a non-metal by transferring electrons. The correct statements are ionic bonds form between metal atoms and nonmetal atoms the less electronegative atoms transfers one or more electrons to the more.
 |
| Notes Ionic Bonds 1 Key Concept Ionic Bonds Form When Electrons Are Ppt Download |
Covalent bonding involves the sharing of electrons between two or more atoms.
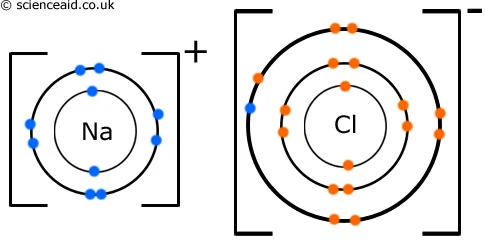
. Electron transfer produces negative ions called anions and positive ions called cations. When atoms lose or gain or share electrons from or to or with their valance shells ionic bonds are formedIonic bonds for when two ions become attracted to each other through. Ionic bonds form when one or. The metal atom forms a cation and.
Ionic bonds form when an anion bonds with a cation. There are primarily two forms of bonding that an atom can participate in. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. This forms two ions Sodium ion with a.
Ionic bond also called electrovalent bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonds form between metal and non metal atoms. How do ionic bonds form. The less electronegative atoms transfers one or more electrons to the more electronegative atom.
When the transfer of electrons occurs an electrostatic attraction between the two ions of. When valence electrons are transferred from one atom to another. Ionic bonds form when one atom has a much higher electronegativity than another. The bond forms when the atom with less than 4 valence electrons Sodium gives its electrons to the atom with more than 4 valence electrons Chlorine.
A ionic bond is formed by gain and loss of electron due to gain of electron anion is formed w View the full answer. Electrostatic force of attraction between metal positive ion and. Learn how to write the chemical formulas for some common ionic compounds including Sodium Chloride Aluminum Oxide and. Such a bond forms when the.
An ionic bond is formed by the complete transfer of some electrons from one atom to another. This causes the electronegative atom to pull electrons from the less electronegative atom. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. So that the outer most energy level of atoms are filled.
How do ionic bonds occur. Ionic bond also called electrovalent bond type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The oppositely charged ions are strongly attracted to each other. What way do ionic bonds form.
Ionic bond is bond that is formed between metals ions always positive ions and non metals ionsalways negative ions.
 |
| Ionic Bond Ck 12 Foundation |
 |
| Ionic Vs Covalent Bonds |
 |
| Ionic Bonding School Of Materials Science And Engineering Unsw Sydney |
 |
| 6 1 Ionic Compounds Pages Learning Goals I Can Explain How Ions And Ionic Compounds Are Formed I Can Describe The Properties Of Ionic Compounds Ppt Download |
 |
| Ionic Bonds Essential Question How Do Ionic Bonds |
Post a Comment for "how do ionic bonds form"